UV Validation Software - Características
UV Validation Software
Validation Operations Are Performed by the Software
Inspection parameters, inspection conditions, and pass/fail criteria can be specified and all operations, from measurements to calculations and pass/fail determinations, can be performed automatically. The ability to save and recall inspection condition files means inspections can be accomplished with a minimum of checking operations.
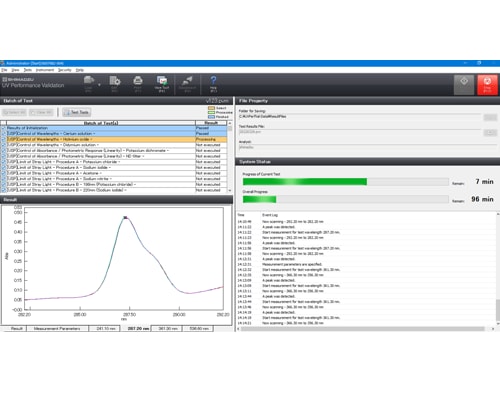
Main Window of UV Validation Software
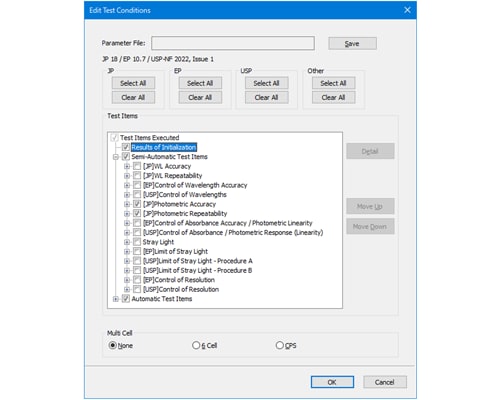
Validation Parameter Settings
Enables Easy Japanese, EU, and US Pharmacopoeia-Compliant Validation
-
Because the Japanese, EU, and US pharmacopoeias each specify different test methods and passing criteria, there is a wide variety of performance parameters that must be checked to achieve compliance with each pharmacopoeia. Checking them all by hand would be time-consuming and the complicated procedure involved would also be prone to operator errors.
The UV validation software does not require any complicated operations, because it already includes all the procedures and pass/fail criteria required for compliance with all three pharmacopoeias preregistered in the software. By using the evaluation function in the software, performance levels specified in each pharmacopoeia can be determined automatically.
Validation also requires documenting results. For example, the operational qualification (OQ) process requires confirming and documenting that the system functions as intended within the expected operating range. Shimadzu field engineers use validation software to prepare evaluation documentation for supporting customer instrument management.
Note: Contact Shimadzu for information about software versions specialized for compliance with a specific pharmacopoeia. 
Validation Software can be Linked to LabSolutions DB/CS Software for Compliance with Data Integrity Requirements
By linking the validation software with LabSolutions DB/CS, measurement data and validation results can be managed together in one place.


